Vitamin B12
The reactions of vitamin B12 and its derivatives with small molecules of biological interest
Figure 1: Structures of Cobalamins found in Humans:
X = H2O aquacobalamin (H2OCbl+)
X = CH3 methylcobalamin (MeCbl)
X = 5′-deoxyadenosine adenosylcobalamin (AdoCbl, “coenzyme B12“)
X = glutathione glutathionylcobalamin (GSCbl)
X = CN cyanocobalamin (CNCbl, “vitamin B12“)
Figure 1 shows the structures of the various forms of vitamin B12 (cobalamins) which are found in the human body. Vitamin B12 derivatives are cofactors in approximately 15 enzyme reactions. Two of these reactions, involving methionine synthase (1) and methylmalonyl-CoA mutase (2), occur in humans and utilize the vitamin B12 derivatives methylcobalamin (MeCbl) and 5′-deoxyadenosylcobalamin (AdoCbl), respectively.
(1) 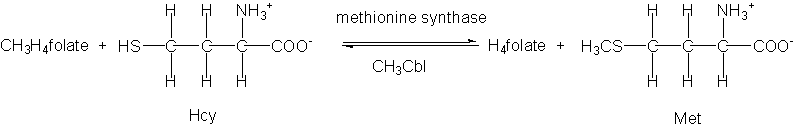
(2) 
In the former reaction, a methyl group is transferred from methyltetrahydrofolate to homocysteine (Hcy) via MeCbl to generate methionine and tetrahydrofolate, and this reaction has received much attention in the medical literature in recent years. It has been shown that patients with high levels of Hcy have a greatly increased risk of suffering a stroke or a heart attack, and there is also increasing evidence that high levels of Hcy are prevalent in sufferers of Alzheimer’s disease and other neurological disorders.
We are especially interested in the thiol derivatives of vitamin B12, thiolatocobalamins, where X = a thiol group (Figure 1). In 2003 we initiated a collaboration with Dr Donald Jacobsen at the Cleveland Clinic Foundation aimed at studying cobalamin processing in endothelial cells. Thus far we have obtained evidence that glutathionylcopbalamin, GSCbl (X = GS, Fig. 1) is present in aortic endothelial cells, and that GSCbl is decomposed during the typical procedures used to extract and purify vitamin B12 derivatives from cells. We have also found that beta-axial ligand exchange occurs during the extraction and purification of other cobalamins from cells, and have modified the extraction procedure to prevent these side reactions from occurring.
More recently we have initiated mechanistic studies on the reactions between vitamin B12 derivatives and ROS/RNS (Reactive Oxygen Species/Reactive Nitrogen Species). ROS/RNS are small molecules generated in biological systems and are typically radicals and/or strong oxidants including nitric oxide, superoxide, the hydroxyl radical, the carbonate radical, peroxynitrite, hydrogen peroxide and related peroxide species. Elevated levels of ROS/RNS are associated with multiple diseases including Alzheimer’s disease Parkinson’s disease, cardiovascular disease, stroke, and cancer. However ROS/RNS also protect by destroying pathogens as part of the innate immune response in addition to being important signaling molecules. An example of this is nitric oxide’s role in smooth muscle relaxation of the cells lining blood vessels.
Others have suggested that nitric oxide may potentially react with B12 in biological systems upon administering high doses of B12. B12 prevents nitric oxide-induced dilation of blood vessels, smooth muscle relaxation and nitric oxide-induced neural tube defects, suggesting that free B12 (most likely cob(II)alamin) can effectively scavenge nitric oxide. Recent studies in our group have focused on elucidating the mechanisms of the reactions of vitamin B12 derivatives with a range of other ROS/RNS, including superoxide, peroxynitrite, and the carbonate radical. Cell studies have also been carried out to probe B12’s ability to prevent intracellular oxidative stress, in collaboration with Andrew McCaddon, John Williams and June Yun. Most ROS/RNS simply oxidize the metal center of cob(II)alamin to cob(III)alamin. However the carbonate and hydroxyl radicals also abstract H atoms from the corrin macrocycle.
